
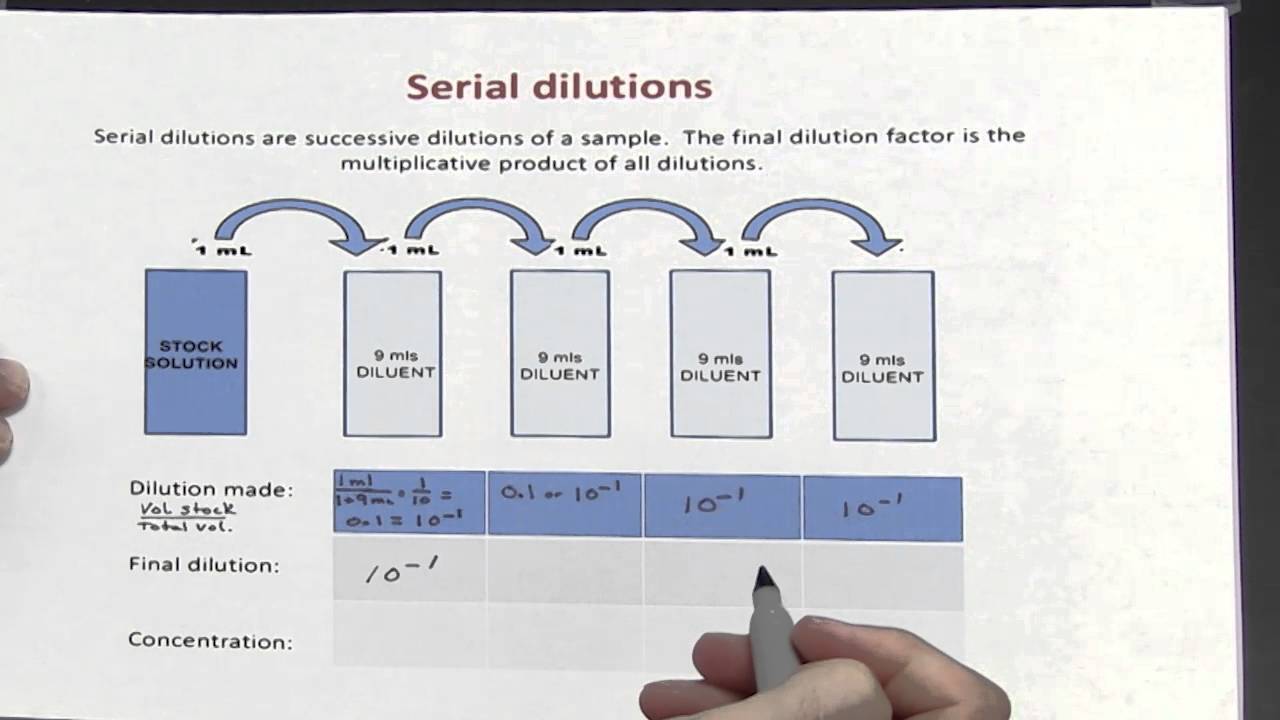
The dilution factor after four dilutions is Repeat the process until you have four tubes.
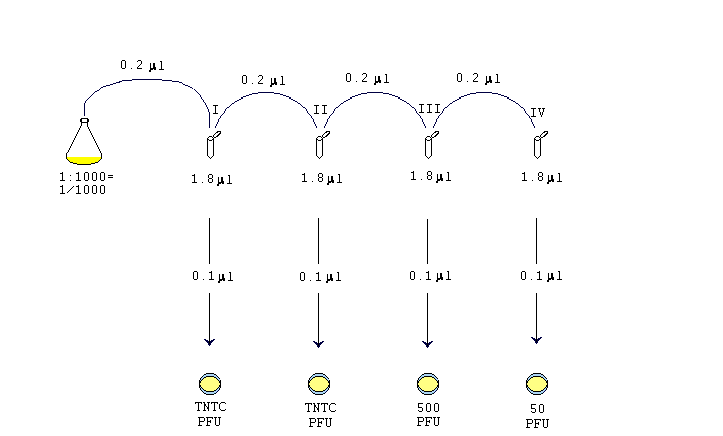
#Serial dilution serial
Then transfer 0.2 mL from Tube 2 to 3.8 mL of diluent in Tube 3 and mix. Serial dilutions have many uses that are mainly related to chemistry and biology. You would transfer 0.2 mL from Tube 1 to 3.8 mL of diluent in Tube 2 and mix. So you multiply each successive dilution by the dilution factor. Remember that serial dilutions are always made by taking a set quantity of the initial dilution and adding it successively to tubes with the same volume. If you did the above dilution four times, what would be the final dilution factor? They are carried out in small sterile test tubes. In this manual, ten-fold serial dilutions are used in titrations of a suspension of Newcastle disease virus to establish the infectivity titre. What is the dilution factor if you add 0.2 mL of a stock solution to 3.8 mL of diluent? A series of ten-fold dilutions is described as ten-fold serial dilutions. #DF = V_i/V_f# = #(1"mL")/(10"mL") = 1/10#. Example 1: The following successive dilutions are applied to a stock solution that is 5.60 M sucrose: Solution. A serial dilution definition is a stepwise series of dilutions that are performed to reduce the concentration of a substance in a solution to a more usable concentration. The dilution factor or the dilution is the initial volume divided by the final volume.įor example, if you add a 1 mL sample to 9 mL of diluent to get 10 mL of solution, In serial dilutions, you multiply the dilution factors for each step. The dilution factor or the dilution is the initial volume divided by the final volume. A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step.


 0 kommentar(er)
0 kommentar(er)
